Nuclear magnetic resonance (NMR) spectroscopy
Nuclear magnetic resonance (MR) spectroscopy is a powerful tool for the determination of structure of chemical compounds and a character of intra- and intermolecular interactions and therefore is widely used for searching information on molecular structure in chemistry, biology, pharmacy and medicine. 1H and 13C spectra remain indispensable in the analysis of organic compounds but NMR has recently experienced a revolution: (1) high-field magnets have dramatically improved the sensitivity and selectivity of NMR spectra; (2) progress in electronics makes the routine observation available for many formerly ‘exotic’ nuclei like oxygen-17 or sulfur-33; (3) advances in new experimental methods allow the observation of numerous tiny effects due to intermolecular interactions, temperature or isotopic substitution.
Intermolecular effects in chemical shifts and spin-spin couplings
Experimental NMR spectra are usually obtained from macroscopic samples in which molecules are influenced by intermolecular interactions. Consequently, the spectral parameters like chemical shifts and spin-spin coupling constants are always modified to some extent by intermolecular interactions. The intermolecular effects in the spectral parameters are significant when molecules are observed in liquids or solids. Similar effects in the gas phase are considerably smaller, usually by an order of magnitude or even less. Sometimes it is convenient to obtain the approximate effect of intermolecular interactions comparing the value of chemical shifts measured in gas and liquid (or solid) medium; then the gas-to-liquid (or gas-to-solid) shifts can be compared for different chemicals and modeled by ab initio calculations [17,21]. The largest intermolecular effects in NMR spectra are present when molecules participate in hydrogen bonds [20] or contain heavy atoms like iodine (I), bromine (Br) etc. [59,69].
Investigations of NMR spectral parameters in the gas phase
Intermolecular interactions also modify the NMR spectral parameters observed in the gas phase. It was first reported by Raynes et al. (J. Chem. Phys. 36, 3481 (1962)) that the magnetic shielding of protons was linearly dependent on density for gaseous samples at. Other magnetic nuclei with small natural abundance were investigated in gaseous samples much later: the first observations of 13C density-dependent shifts were described in 1977 [7,8], 17O chemical shifts in 2001 [46] and 33S shifts in 2002 [49]. Generally, it has been clear that the isotropic spin-spin coupling is also sensitive on intermolecular interactions but in gases such effects were relatively weak and formerly they could be observed when molecules were transferred from liquids to vapors. Jameson and Reger (J. Phys. Chem. 75, 437 (1971)) made the first measurements of spin-spin coupling as a function of gas density in SiF4. In our laboratory it was shown that the density dependence of spin-spin coupling is widespread and can be observed for numerous chemical compounds [42,49,50,51,59-63].
Measurements of magnetic shielding and spin-spin coupling constants
for isolated molecules
Investigations of NMR spectral parameters of gaseous molecules permit the exact evaluation of intermolecular contributions and consequently allow one to determine the parameters free from intermolecular interactions which are equivalent to the spectral parameters of isolated molecules. In our Laboratory of NMR Spectroscopy we have measured numerous shielding constants free from intermolecular effects (σ0), e.g. 1H and 13C for 1,2-13C-enriched acetylene [42], 17O for CO, CO2, N2O, SO2 and OCS [46,58], 33S for SF6, SO2 and OCS [49,52,58] and many others. We found that the application of gaseous solvent as a matrix will allow us to extend such investigations to cover chemicals which are liquid at standard temperature and pressure. Following the above idea it was possible to determine appropriate shielding parameters for acetonitrile [51, 54], benzene [63], sevoflurane [65], methyl iodide [59] and some others chemicals. The isotropic spin-spin coupling between magnetic nuclei is also modified by intermolecular interactions in gases. We could observe a strong density dependence of the coupling 19F-13C nuclei in CD3F [50] and we measured the 1J0 coupling constant. Many other spin-spin couplings for isolated molecules were also determined together with appropriate shielding parameters. Such experimental data are important for comparison with the results of quantum-chemical calculations of magnetic shielding and spin-spin coupling as the appropriate calculations are most frequently performed for isolated molecules. Exact theoretical studies also account for the change of NMR spectral parameters due to the increase of temperature which makes the comparison of experimental [42] and theoretical (Jaszuński and Ruud, K. Chem. Phys. Lett. 336, 473 (2001)) spectra really excellent (see below the 13C NMR spectrum of acetylene-13C2 as an example). It proves that the future analysis of NMR spectra can totally be based on the use of ab initio calculations and fully performed by computers.
Experimental and theoretical results for an isolated acetylene-13C2 molecule
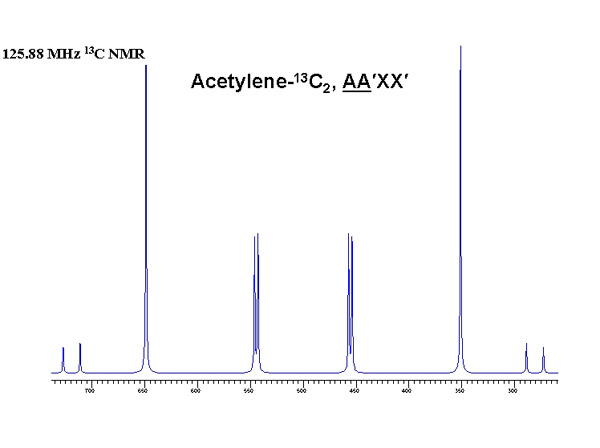
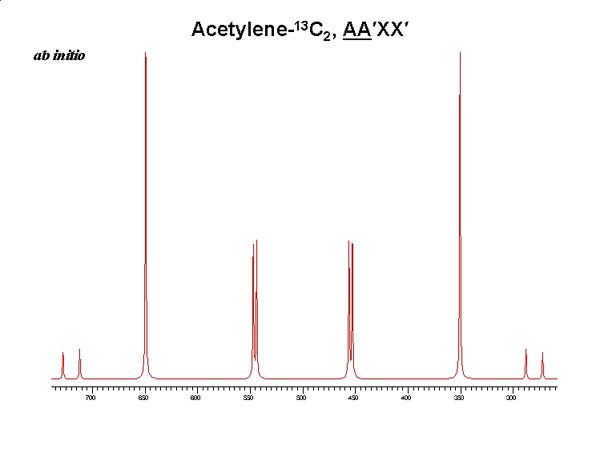
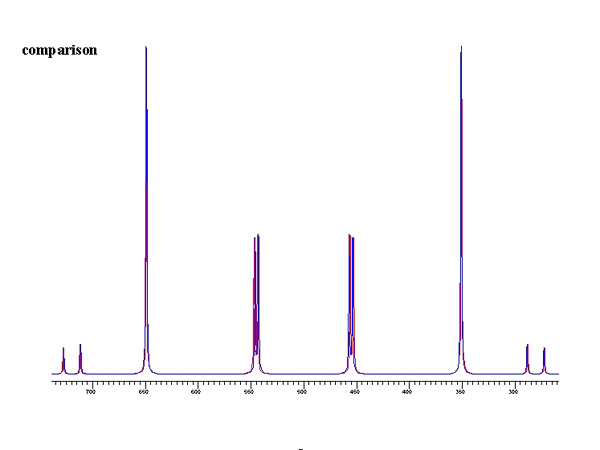
Evaluation of nuclear magnetic dipole moments from NMR spectra
The magnitudes of nuclear magnetic moments are crucial for nuclear physics, they are also present in molecular spectroscopies as important physical constants. There are currently no nuclear models that can predict the large variation in the nuclear dipole moments for different nuclides, and the values of the moments have to be determined experimentally. At present, only the magnetic moment of 1H is known with high accuracy from direct measurements. The magnetic moments of other nuclides can be determined if the shielding constants, absolute resonance frequencies and magnetic moments are compared for a molecule containing protons and the investigated nuclide [70]. The above procedure was already successfully applied to determine nuclear dipole moments of following nuclides: 10B, 11B, 13C, 14N, 15N, 17O, 19F, 29Si, 31P, 33S, 35Cl, 37Cl, 73Ge, 207Pb, 209Bi [64,68,73,79,83,86,89,90]. We found that the new magnetic moments were different by an order of magnitude or more from the previous data. It is also important that for the first time the new nuclear magnetic moments are consistent with the basic experimental NMR parameters, i.e. shielding and resonance frequencies.
Isotopic effects
Isotopic effects are often found in NMR chemical shifts and spin-spin coupling. They occur as a result of the small change of molecular geometry when two isotopologues are observed at the same temperature. NMR spectra performed for liquid samples give only approximate evaluation of isotope effects because intermolecular interactions can significantly change the result of measurements. The real isotopic effects are available if the spectral parameters free from intermolecular interactions are determined for investigated isotopologues [50]. Such experimental results are suitable for the detail analysis of intramolecular dynamics.
Measurements of magnetic shielding
The method of 1H and 13C shielding measurements available on a standard NMR spectrometer was recently presented in ref. [75]. It is based on the magnetic shielding of an isolated helium-3 atom, its resonance frequency and determined according to Eq. (1):
| |
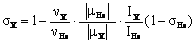
|
(1)
|
where ν, μ and I are the resonance frequencies, the nuclear magnetic moments and the spin numbers of helium-3 and another observed nucleus (X), respectively. Eq. (1) can be used for the shielding measurement of any nucleus when the magnetic moment of the given nucleus is accurately known. The isolated helium-3 atom serves as the primary reference standard of shielding and can be potentially applied to all the magnetic nuclei. Moreover the vantage point of the absolute shielding scale can be transferred from helium-3 to another reference standard, such as deuterated lock solvent, and one can conveniently use in practice this procedure as described by Eq. (2):
| |
σX = 1– CX(νX/νD)(1– σ*D)
|
(2)
|
where σ* represents the shielding of deuterons in selected liquid solvent [75] and CX parameters are known for the majority of light magnetic nuclei like 1H, 2H, 11B, 13C, 15N, 17O, 19F, 29Si and 31P [80].
Alternative standardization of NMR spectra
As shown in ref. [75], the chemical shifts can be successfully replaced by the measurement of shielding parameters and this new method of standardization of NMR spectra has numerous distinct advantages.
|
(1)
|
It unifies multinuclear methods into one NMR spectroscopy because the values of magnetic shielding have the same molecular meaning independently of observed nuclei.
|
|
(2)
|
There is no need to use any additional reference standard if NMR experiment is performed with deuterated lock solvent.
|
|
(3)
|
Experimental shielding parameters can be easily converted into chemical shifts if necessary and the shielding of selected reference compound is known.
|
|
(4)
|
The precision of shielding measurements is in every case exactly the same as for the appropriate chemical shifts because it is based on the same reading of resonance frequencies.
|
|
(5)
|
The experimental shielding values determined in this way are ready for direct comparison with the results of quantum-chemical calculations for the same molecular objects.
|
|
(6)
|
The new method permits the determination of first order isotope effects in shielding as it was already shown for hydrogen isotopologues [82].
|
|
(7)
|
Shielding measurements performed using any external or internal deuterated solvent give practically the same results.
|
|
(8)
|
The measurement of shielding can be easily extended to cover MAS NMR experiments as it was shown for 13C spectra [76].
|
All the above conclusions are illustrated with the results of our recent experimental studies [75, 76, 79, 80, 82].
|